Products
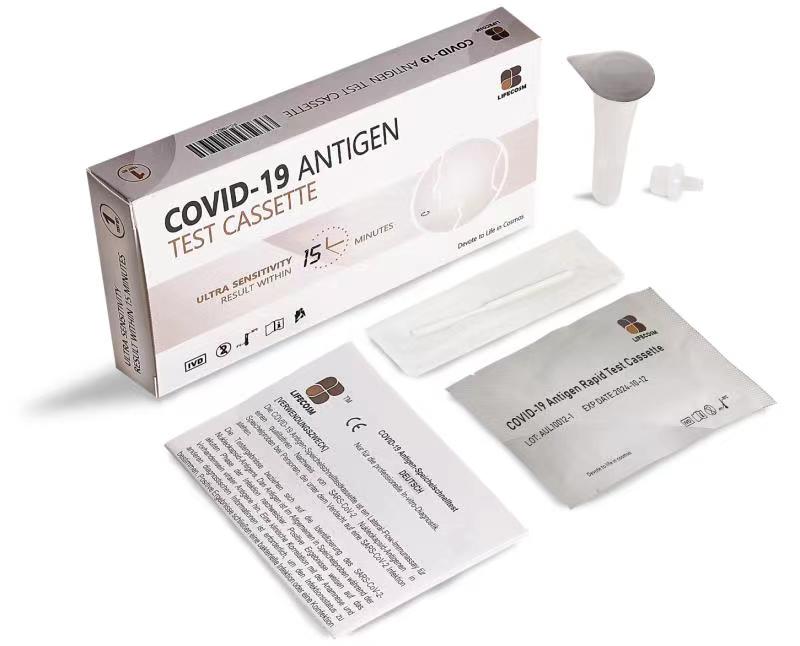
Lifecosm COVID-19 Antigen Test Cassette Antigen test
COVID-19 Antigen Test Cassette
| Summary | Detection of specific Antigen of Covid-19 within 15 minutes |
| Principle | One-step immunochromatographic assay |
| Detection Targets | COVID-19 Antigen |
| Sample | oropharyngeal swab, nasal swab, or saliva |
| Reading time | 10~ 15 minutes |
| Quantity | 1 box (kit) = 25 devices (Individual packing) |
| Contents | 25 Test Cassettes: each cassette with desiccant in individual foil pouch 25 Sterilized Swabs: single use swab for specimen collection
25 Extraction Tubes: containing 0.4mL of extraction reagent 25 Dropper Tips 1 Work Station 1 Package Insert |
|
Caution |
Use within 10 minutes after openingUse appropriate amount of sample (0.1 ml of a dropper)
Use after 15~30 minutes at RT if they are stored under cold circumstances Consider the test results as invalid after 10 minutes |
COVID-19 Antigen Test Cassette
The COVID-19 Antigen Rapid Test Cassette is a lateral flow immunoassay intended for the qualitative detection SARS-CoV-2 nucleocapsid antigens in nasopharyngeal swab, oropharyngeal swab, nasal swab, or saliva from individuals who are suspected of COVID-19 by their healthcare provider.
Results are for the identification of SARS-CoV-2 nucleocapsid antigen. Antigen is generally detectable in oropharyngeal swab, nasal swab, or saliva during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary for patient management.
The COVID-19 Antigen Rapid Test Cassette intended for use by medical professionals or trained operators who are proficient in performing lateral flow tests. The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulation.
PRINCIPLE
The COVID-19 Antigen Rapid Test Cassette is a lateral flow immunoassay based on the principle of the double-antibody sandwich technique. SARS-CoV-2 nucleocapsid protein monoclonal antibody conjugated with color microparticles is used as detector and sprayed on conjugation pad. During the test, SARS-CoV-2 antigen in the specimen interacts with SARS-CoV-2 antibody conjugated with color microparticles making antigen-antibody labeled complex. This complex migrates on the membrane via capillary action until the test line, where it will be captured by the pre-coated SARS-CoV-2 nucleocapsid protein monoclonal antibody. A colored test line (T) would be visible in the result window if SARS-CoV-2 antigens are present in the specimen. Absence of the T line suggests a negative result. The control line (C) is used for procedural control, and should always appear if the test procedure is performed properly.
[SPECIMEN]
Specimens obtained early during symptom onset will contain the highest viral titers; specimens obtained after five days of symptoms are more likely to produce negative results when compared to an RT-PCR assay. Inadequate specimen collection, improper specimen handling and/or transport may yield false results; therefore, training in specimen collection is highly recommended due to the importance of specimen quality to obtain accurate test results.
Acceptable specimen type for testing is a direct swab specimen or a swab in viral transport media (VTM) without denaturing agents. Use freshly collected direct swab specimens for best test performance.
Prepare the extraction tube according to the Test Procedure and use the sterile swab provided in the kit for specimen collection.
Nasopharyngeal Swab Specimen Collection