Products
Lifecosm Anaplasma Ab Rapid Test Kit
Anaplasma Phagocytophilum Ab Test Kit
|
Anaplasma Phagocytophilum Ab Test Kit |
|
| Catalog number | RC-CF26 |
| Summary | Detection of specific antibodies of Anaplasmawithin 10 minutes |
| Principle | One-step immunochromatographic assay |
| Detection Targets | Anaplasma antibodies |
| Sample | Canine whole blood, serum or plasma |
| Reading time | 5~ 10 minutes |
| Sensitivity | 100.0 % vs. IFA |
| Specificity | 100.0 % vs. IFA |
| Limit of Detection | IFA Titer 1/16 |
| Quantity | 1 box (kit) = 10 devices (Individual packing) |
| Contents | Test kit, Buffer bottle, and Disposable droppers |
|
Caution |
Use within 10 minutes after openingUse appropriate amount of sample (0.01 ml of a dropper)
Use after 15~30 minutes at RT if they are stored under cold circumstances Consider the test results as invalid after 10 minutes |
Information
The bacterium Anaplasma phagocytophilum (formerly Ehrilichia phagocytophila) may cause infection in several animal species including human. The disease in domestic ruminants is also called tick-borne fever (TBF), and has been known for at least 200 years. Bacteria of the family Anaplasmataceae are gram-negative, nonmotile, coccoid to ellipsoid organisms, varying in size from 0.2 to 2.0um diameter. They are obligate aerobes, lacking a glycolytic pathway, and all are obligate intracellular parasites. All species in the genus Anaplasma inhabit membrane-lined vacuoles in immature or mature hematopoietic cells of mammalian host. A phagocytophilum infects neutrophils and the term granulocytotropic refers to infected neutrophils. Rarely organisms, have been found in eosinophils.
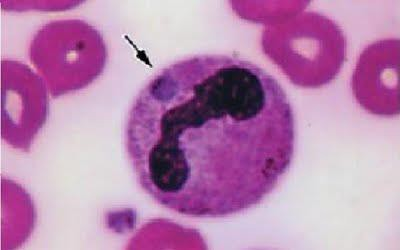
Anaplasma phagocytophilum
Symptoms
Common clinical signs of canine anaplasmosis include high fever, lethargy, depression and polyarthritis. Neurologic signs (ataxia, seizures and neck pain) can also be seen. Anaplasma phagocytophilum infection is seldom fatal unless complicated by other infections. Direct losses, crippling conditions and production losses have been observed in lambs. Abortion and impaired spermatogenesis in sheep and cattle have been recorded. The severity of the infection is influenced by several factors, such as variants of Anaplasma phagocytophilum involved, other pathogens, age, immune status and condition of the host, and factors such as climate and management. It should be mentioned that clinical manifestations in humans range from a mild selflimited flu-like illness, to a life-threatening infection. However, most human infections probably result in minimal or no clinical manifestations.
Transmission
Anaplasma phagocytophilum is transmitted by ixodid ticks. In the United States the principal vectors are Ixodes scapularis and Ixodes pacificus, while Ixode ricinus has been found to be the main exophilic vector in Europe. Anaplasma phagocytophilum is transstadially transmitted by these vector ticks, and there is no evidence of transovarial transmission. Most studies to date that have investigated the importance of mammalian hosts of A. phagocytophilum and its tick vectors have focused on rodents but this organism has a wide mammalian host range, infecting domesticated cats, dogs, sheep, cows, and horses.

Diagnosis
Indirect immunofluorescence assay is the principal test used to detect infection. The acute and convalescent phase serum samples can be evaluated to look for a four-fold change in antibody titer to Anaplasma phagocytophilum. Intracellular inclusions (morulea) are visualized in granulocytes on Wright or Gimsa stained blood smears. Polymerase chain reaction(PCR) methods are used to detect Anaplasma phagocytophilum DNA.
Prevention
No vaccine is available to prevent Anaplasma phagocytophilum infection. Prevention relies on avoiding exposure to the tick vector (Ixodes scapularis, Ixodes pacificus, and Ixode ricinus) from spring through fall, prophylatic use of antiacaricides, and prophylactic use of doxycycline or tetracycline when visiting Ixodes scapularis, Ixodes pacificus, and Ixode ricinus tick-endemic regions.