Products
Renewable Design for Nasopharyngeal Np Swab - Lifecosm CHW Ag/Anaplasma Ab/E.canis Ab/LSH Ab Test Kit Veterinary medicine – Lifecosm
Renewable Design for Nasopharyngeal Np Swab - Lifecosm CHW Ag/Anaplasma Ab/E.canis Ab/LSH Ab Test Kit Veterinary medicine – Lifecosm Detail:
CHW Ag/Anaplasma Ab/E.canis Ab/LSH Ab Test Kit Canine Heartworm Ag/Anaplasma Ab /Ehrlichia canis Ab/Leishmania Ab test kit
| Catalog number | RC-CF31 |
| Summary |
Detection of Canine Dirofilaria immitis antigens, Anaplasma antibodies, E. canis antibodies and LSH antibodies within 10 minutes |
| Principle | One-step immunochromatographic assay |
| Detection Targets | CHW Ag : Dirofilaria immitis antigens Anapalsma Ab : Anaplasma antibodiesE. canis Ab : E. canis antibodies
LSH Ab : L. chagasi, L. infantum, and L. donovani antiboies |
| Sample | Canine Whole Blood, Plasma or Serum |
| Reading time | 10 minutes |
| Quantity | 1 box (kit) = 10 devices (Individual packing) |
| Contents | Test kit, Buffer bottle, and Disposable dropper |
| Storage | Room Temperature (at 2 ~ 30℃) |
| Expiration | 24 months after manufacturing |
|
Caution |
Use within 10 minutes after openingUse appropriate amount of sample (0.01 ml of a dropper)
Use after 15~30 minutes at RT if they are stored under cold circumstances Consider the test results as invalid after 10 minutes |
Information
Adult heartworms grow several inches in length and reside in the pulmonary arteries where it can obtain enough nutrients. The heartworms inside the arteries trigger inflammation and form hematoma. The heart, then, should pump more often than before as the heartworms increase in number, blocking the arteries.
When infection deteriorates (over 25 heartworms exist in a 18 kg dog), the heartworms move into the right atrium, blocking the flow of blood.
When the number of heartworms reaches more than 50, they could occupy
atriums and ventricles.
When infected with over 100 heartworms in the right part of the heart, the dog loses the function of the heart and eventually dies. This fatal
phenomenon is called as “Caval Syndrom.”
Unlike other parasites, the heartworms lay small insects called as microfilaria. Microfilaria in mosquito moves into a dog when the mosquito sucks blood from the dog. The heartworms that can survive in the host for 2 years die if they do not move into another host within that period. The parasites residing in a pregnant dog can infect its embryo.
Early examination of heartworms is very important in eliminating them. Heartworms go through several steps such as L1, L2, L3 including the transmission stage through mosquito to become adult heartworms.
Heartworms in mosquito
Microfilaria in mosquito grows into L2 and L3 parasites able to infect dogs in several weeks. The growth depends on the weather. Favorable temperature for the parasite is over 13.9℃.
When an infected mosquito bites a dog, microfilaria of L3 penetrates into its skin. In the skin, the microfilaria grows into L4 for 1~2 weeks. After residing in the skin for 3 months, L4 develops into L5, which moves into blood.
L5 as the form of adult heartworm enters the heart and pulmonary arteries where 5~7 months later heartworms lay insects.
Diagnosis
The disease history and clinical data of a sick dog, and various diagnostic methods should be considered in diagnosing the dog. For example, X-ray, ultrasound scan, blood examination, detection of microfilaria and, in worst case, autopsy are required.
Serum examination;
Detection of antibodies or antigens in the blood
Antigen examination;
This focuses on detecting the specific antigens of female adult heartworms. The examination is carried out in the hospital and its success rate is high. Test kits available on the market are designed to detect 7~8 month-old adult heartworms so that heartworms younger than 5 months are hard to detect.
Treatment
The infection of heartworms is successfully cured in most cases. To eliminate all heartworms, the use of medicines is the best way. Early detection of the heartworms raises the success rate of the treatment. However, in the late stage of infection, complication can occur, making the treatment more difficult.
Information
The bacterium Anaplasma phagocytophilum (formerly Ehrilichia phagocytophila) may cause infection in several animal species including human. The disease in domestic ruminants is also called tick-borne fever (TBF), and has been known for at least 200 years. Bacteria of the family Anaplasmataceae are gram-negative, nonmotile, coccoid to ellipsoid organisms, varying in size from 0.2 to 2.0um diameter. They are obligate aerobes, lacking a glycolytic pathway, and all are obligate intracellular parasites. All species in the genus Anaplasma inhabit membrane-lined vacuoles in immature or mature hematopoietic cells of mammalian host. A phagocytophilum infects neutrophils and the term granulocytotropic refers to infected neutrophils. Rarely organisms, have been found in eosinophils.
Anaplasma phagocytophilum
Symptoms
Common clinical signs of canine anaplasmosis include high fever, lethargy, depression and polyarthritis. Neurologic signs (ataxia, seizures and neck pain) can also be seen. Anaplasma phagocytophilum infection is seldom fatal unless complicated by other infections. Direct losses, crippling conditions and production losses have been observed in lambs. Abortion and impaired spermatogenesis in sheep and cattle have been recorded. The severity of the infection is influenced by several factors, such as variants of Anaplasma phagocytophilum involved, other pathogens, age, immune status and condition of the host, and factors such as climate and management. It should be mentioned that clinical manifestations in humans range from a mild selflimited flu-like illness, to a life-threatening infection. However, most human infections probably result in minimal or no clinical manifestations.
Transmission
Anaplasma phagocytophilum is transmitted by ixodid ticks. In the United States the principal vectors are Ixodes scapularis and Ixodes pacificus, while Ixode ricinus has been found to be the main exophilic vector in Europe. Anaplasma phagocytophilum is transstadially transmitted by these vector ticks, and there is no evidence of transovarial transmission. Most studies to date that have investigated the importance of mammalian hosts of A. phagocytophilum and its tick vectors have focused on rodents but this organism has a wide mammalian host range, infecting domesticated cats, dogs, sheep, cows, and horses.

Diagnosis
Indirect immunofluorescence assay is the principal test used to detect infection. The acute and convalescent phase serum samples can be evaluated to look for a four-fold change in antibody titer to Anaplasma phagocytophilum. Intracellular inclusions (morulea) are visualized in granulocytes on Wright or Gimsa stained blood smears. Polymerase chain reaction(PCR) methods are used to detect Anaplasma phagocytophilum DNA.
Prevention
No vaccine is available to prevent Anaplasma phagocytophilum infection. Prevention relies on avoidnig exposure to the tick vector (Ixodes scapularis, Ixodes pacificus, and Ixode ricinus) from spring through fall, prophylatic use of antiacaricides, and prophylactic use of doxycycline or tetracycline when visiting Ixodes scapularis, Ixodes pacificus, and Ixode ricinus tick-endemic regions.
Information
Ehrlichia canis is a small and rod shaped parasites transmitted by the brown dog tick, Rhipicephalus sanguineus. E. canis is the cause of classical ehrlichiosis in dogs. Dogs may be infected by several Ehrlichia spp. but the most common one causing canine ehrlichiosis is E. canis.
E. canis has now been known to have spread all over the United States, Europe, South America, Asia and the Mediterranean.
Infected dogs that are not treated can become asymptomatic carriers of the disease for years and eventually die from massive hemorrhage.
Symptoms
Ehrlichia canis infection in dogs is divided into 3 stages;
ACUTE PHASE: This is generally a very mild phase. The dog will be listless, off food, and may have enlarged lymph nodes. There may be fever as well but rarely does this phase kill a dog. Most clear the organism on their own but some will go on to the next phase.
SUBCLINICAL PHASE: In this phase, the dog appears normal. The organism has sequestered in the spleen and is essentially hiding out there.
CHRONIC PHASE: In this phase the dog gets sick again. Up to 60% of dogs infected with E. canis will have abnormal bleeding due to reduced platelets numbers. Deep inflammation in the eyes called “uveitis” may occur as a result of the long term immune stimulation. Neurologic effects may also be seen.
Diagnosis and treatment
Definitive diagnosis of Ehrlichia canis requires visualization of morula within monocytes on cytology, detection of E. canis serum antibodies with the indirect immunofluorescence antibody test (IFA), polymerase chain reaction (PCR) amplification, and/or gel blotting (Western immunoblotting).
The mainstay of prevention of canine ehrlichiosis is tick control. The drug of choice for treatment for all forms of ehrlichiosis is doxycycline for at least one month. There should be dramatic clinical improvement within 24-48 hours following initiation of treatment in dogs with acute-phase or mild chronic-phase disease. During this time, platelet counts begin to increase and should be normal within 14 days after initiation of treatment.
After infection, it is possible to become re-infected; immunity is not lasting after a previous infection.
Prevention
The best prevention of ehrlichiosis is to keep dogs free of ticks. This should include checking the skin daily for ticks and treating dogs with tick control. Since ticks carry other devastating diseases, such as Lyme disease, anaplasmosis and Rocky Mountain spotted fever, it’s important to keep dogs tick-free.
Information
Leishmaniasis is a major and severe parasitic disease of humans, canines and felines. The agent of leishmaniasis is a protozoan parasite and belongs to the leishmania donovani complex. This parasite is widely distributed in temperate and subtropical countries of Southern Europe, Africa, Asia, South America and Central America. Leishmania donovani infantum (L. infantum) is responsible for the feline and canine disease in Southern Europe, Africa, and Asia. Canine Leishmaniasis is a severe progressive systemic disease. Not all dogs develop clinical disease after inoculation with the parasites. The development of clinical disease is dependent on the type of immune response that individual animals have
against the parasites.
Symptoms
In Canine
Both visceral and cutaneous manifestations may be found simultaneously in dogs; unlike humans, separate cutaneous and visceral syndromes are not seen. The clinical signs are variable and can mimic other infections. Asymptomatic infections can also occur. Typical visceral signs may include fever (which can be intermittent), anemia, lymphadenopathy, splenomegaly, lethargy, decreased exercise tolerance, weight loss, and a decreased appetite. Less common visceral signs include diarrhea, vomiting, melena, glomerulonephritis, liver failure, epistaxis, polyuria-polydipsia, sneezing, lameness (due to polyarthritis or myositis), ascites, and chronic colitis.
In Feline
Cats are rarely infected. In most infected cats, the lesions are limited to crusted cutaneous ulcers, usually found on the lips, nose, eyelids, or pinnae. Visceral lesions and signs are rare.
Life cycle
The life cycle is completed in two hosts. A vertebrate host and an invertebrate host (sand fly). The female sand fly feeds on vertebrate host and ingests amastigotes. Flagellated promastigotes develop in the insect. The promastigotes are injected into the vertebrate host during feeding of the sandfly. The promastigotes develop into amastigotes and multiply primarily in the macrophages. Multiplication within the macrophages of the skin, mucosa and viscera, causes cutaneous, mucosal and visceral leishmaniasis respectively
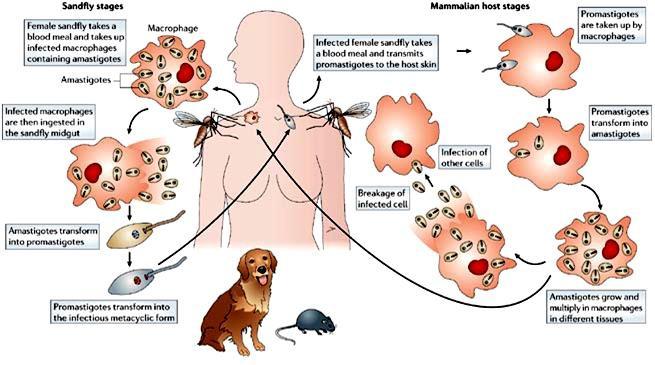
Diagnosis
In dogs, leishmaniasis is usually diagnosed by direct observation of the parasites, using Giemsa or proprietary quick stains, in smears from lymph node, spleen, or bone marrow aspirates, tissue biopsies, or skin scrapings from lesions. Organisms may also be found in ocular lesions, particularly in granulomas. The amastigotes are round to oval parasites, with a round basophilic nucleus and a small rodlike kinetoplast. They are found in macrophages or freed from ruptured cells. Immunohistochemistry and polymerase chain reaction (PCR) techniques are also used.
Prevention
The drugs most commonly used are: Meglumine Antimoniate associated with Allopurinol, Aminosidine, and recently, Amphotericin B. All these drugs require a multiple dose regimen, and this will depend on the patient’s condition and owner cooperation. It is suggested that maintenence treatment should be kept with allopurinol, because it is not possible to ensure that dogs will not relapse if treatment is discontinued. The use of collars containing insecticides, shampoos or sprays effective to protect dogs from sandfly bites must be continuously used for all patients under treatment. The vector control is one of the most important aspects of disease control.
The sandfly is vulnerable to the same insecticides as the malaria vector.
Product detail pictures:
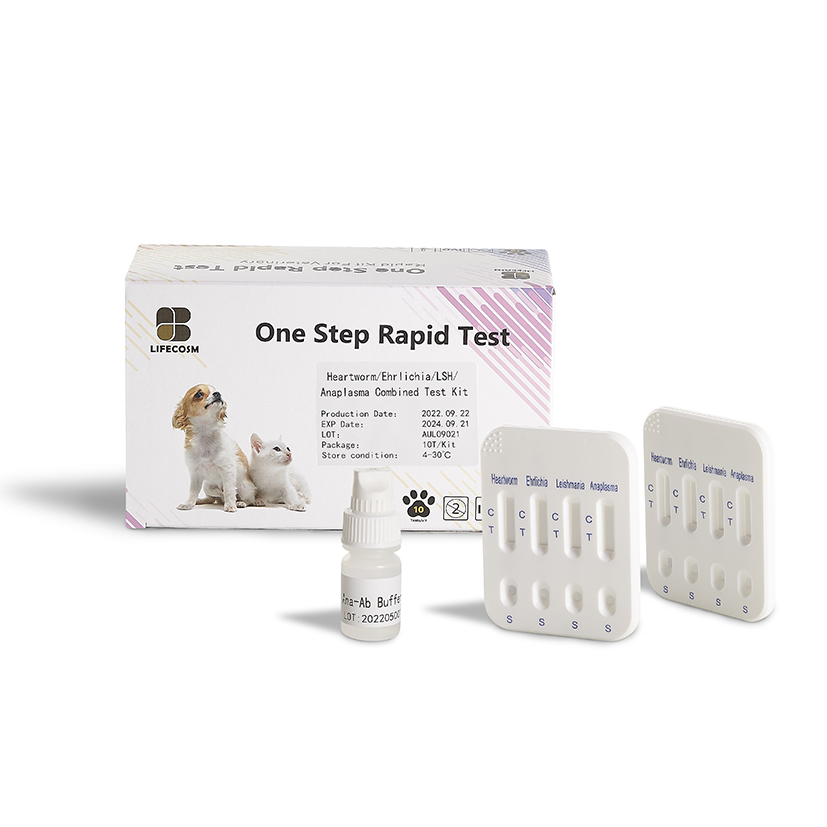


Related Product Guide:
We keep on with the basic principle of quality to start with, support very first, continuous improvement and innovation to meet the customers for your management and zero defect, zero complaints as the quality objective. To great our service, we offer the items with all the superior top quality at the reasonable selling price for Renewable Design for Nasopharyngeal Np Swab - Lifecosm CHW Ag/Anaplasma Ab/E.canis Ab/LSH Ab Test Kit Veterinary medicine – Lifecosm , The product will supply to all over the world, such as: Moscow, Frankfurt, Roman, With good quality, reasonable price and sincere service, we enjoy a good reputation. Products are exported to South America, Australia, Southeast Asia and so on. Warmly welcome customers at home and abroad to cooperate with us for the brilliant future.
It is a very good, very rare business partners, looking forward to the next more perfect cooperation!